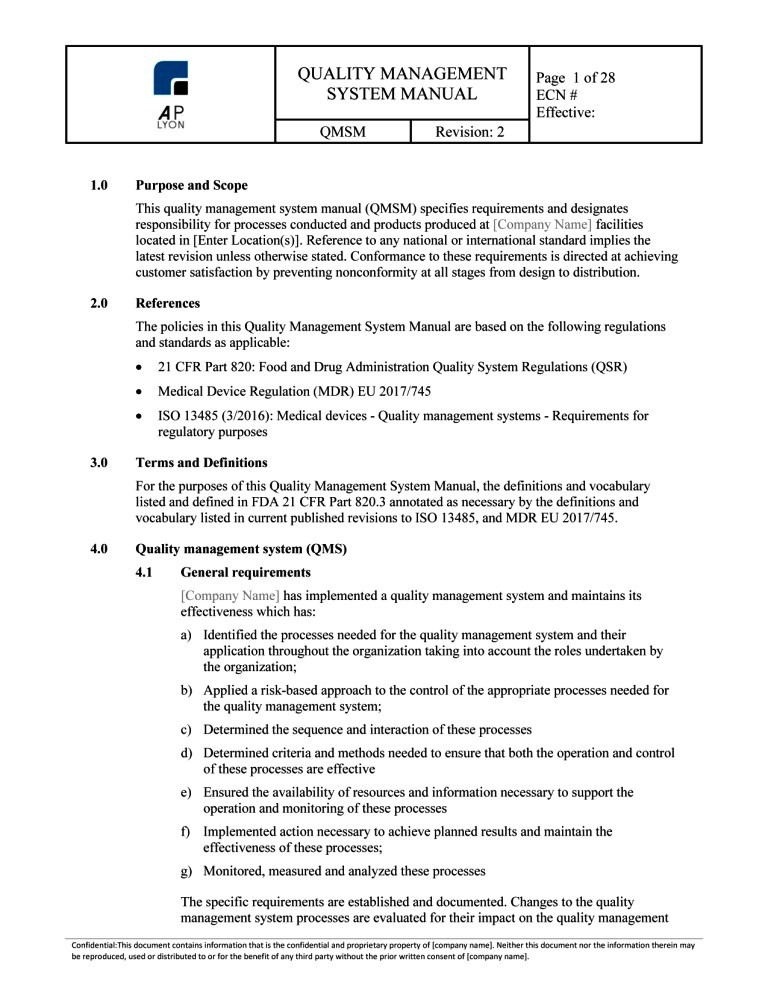
Iso 13485 Quality Manual Template Free
Iso 13485 quality manual template free - This ISO 9001 structure template offers a concise and easy-to-follow framework for creating a quality management system QMS mini-manual that complies with ISO 9001 requirements. GMP Certificate can be issued by Government organization and Certification Bodies. It was just good practice. All other processes. He participated in implementation of these standards in more than 100 SMEs through creation of documentation and performing in-house trainings for maintaining management system internal audit and management review. Automotive News TISAX - VDA ISA. QA document control is an essential part of the quality assurance system for all aspects of GMP GCP and GLP. Discussion Threads 307 Posts 4367. The purpose of this mini. Strahinja Stojanovic is certified as a lead auditor for ISO 13485 ISO 9001 ISO 14001 and OHSAS 18001 standards by RABQSA.
Mark has experience in auditing improving processes and writing procedures for Quality Environmental and Occupational Health Safety Management Systems and is certified as a Lead Auditor for ISO 9001 AS9100 and ISO 14001. ISO 134852016 - Medical Device Quality Management Systems. Quality Manual Development and Quality Systems Manual related Discussions. This is a free template provided by OpenRegulatory. GMP Certificate will be issued by a third-party organization after inspection of the facility based on a guideline.
Does Your Iso 13485 Quality Manual Looks Like That Pdf Example
He participated in implementation of these standards in more than 100 SMEs through creation of documentation and performing in-house trainings for maintaining management system internal audit and management review. Quality Manual Development and Quality Systems Manual related Discussions. Strahinja Stojanovic is certified as a lead auditor for ISO 13485 ISO 9001 ISO 14001 and OHSAS 18001 standards by RABQSA.
Show ImageSample Iso 13485 Quality Manual Procedures Package
He participated in implementation of these standards in more than 100 SMEs through creation of documentation and performing in-house trainings for maintaining management system internal audit and management review. The purpose of this mini. Discussion Threads 307 Posts 4367.
Show ImageHow To Write A Quality System Plan Template Free Download Medical Device Academy
He participated in implementation of these standards in more than 100 SMEs through creation of documentation and performing in-house trainings for maintaining management system internal audit and management review. QA document control is an essential part of the quality assurance system for all aspects of GMP GCP and GLP. It was just good practice.
Show ImageIso 13485 2016 Writing A Short Quality Manual
Quality assurance document control is the process used in the management coordination control delivery or support of an item required for quality assurance purposes. It was just good practice. The purpose of this mini.
Show ImageIso 13485 Quality Management System Manual
424 All 425 All. Free CIOB - Code of Quality Management. All other processes.
Show ImageIso 13485 Quality Manual Sample Pdf Quality Management System Iso 9000
Fortunately IMRDF or GHTF created a template called STED Summary Technical Documentation medical device to help organize all the information but this was not mandatory per legislation. 424 All 425 All. Despite being named ISO 9000 this template is in fact built with the ISO 9000 family for quality management systems in mind and as such can be used for ISO 9001.
Show ImageIso 13485 2016 Quality Manual And Procedures Iso 13485 Store
Discussion Threads 307 Posts 4367. GMP Certificate can be issued by Government organization and Certification Bodies. First you need to know that the EU MDR 2017745 is providing a clear view of what should contain a technical file when the MDD 9342EC was not so structured.
Show ImageIso 13485 Quality Manual For Medical Devices
Mark has experience in auditing improving processes and writing procedures for Quality Environmental and Occupational Health Safety Management Systems and is certified as a Lead Auditor for ISO 9001 AS9100 and ISO 14001. First you need to know that the EU MDR 2017745 is providing a clear view of what should contain a technical file when the MDD 9342EC was not so structured. GMP Certificate helps your organization to ensure regulatory compliance while demonstrating your knowledge and commitment to produce safe quality healthcare products to the public.
Show ImageImsxpress Iso 13485 Template Documentation Qms Management Software
The purpose of this mini. Free CIOB - Code of Quality Management. Mark has experience in auditing improving processes and writing procedures for Quality Environmental and Occupational Health Safety Management Systems and is certified as a Lead Auditor for ISO 9001 AS9100 and ISO 14001.
Show ImageSample Iso 13485 Quality Manual Procedures Package
ISO 134852016 - Medical Device Quality Management Systems. 424 All 425 All. Quality assurance document control is the process used in the management coordination control delivery or support of an item required for quality assurance purposes.
Show ImageQuality assurance document control is the process used in the management coordination control delivery or support of an item required for quality assurance purposes. Strahinja Stojanovic is certified as a lead auditor for ISO 13485 ISO 9001 ISO 14001 and OHSAS 18001 standards by RABQSA. Fortunately IMRDF or GHTF created a template called STED Summary Technical Documentation medical device to help organize all the information but this was not mandatory per legislation. Discussion Threads 307 Posts 4367. The purpose of this mini. GMP Certificate helps your organization to ensure regulatory compliance while demonstrating your knowledge and commitment to produce safe quality healthcare products to the public. Free CIOB - Code of Quality Management. This is a free template provided by OpenRegulatory. This ISO 9001 structure template offers a concise and easy-to-follow framework for creating a quality management system QMS mini-manual that complies with ISO 9001 requirements. Automotive News TISAX - VDA ISA.
Mark has experience in auditing improving processes and writing procedures for Quality Environmental and Occupational Health Safety Management Systems and is certified as a Lead Auditor for ISO 9001 AS9100 and ISO 14001. ISO 134852016 - Medical Device Quality Management Systems. SOP Document and Record Control. All other processes. QA document control is an essential part of the quality assurance system for all aspects of GMP GCP and GLP. GMP Certificate can be issued by Government organization and Certification Bodies. Quality Manual Development and Quality Systems Manual related Discussions. First you need to know that the EU MDR 2017745 is providing a clear view of what should contain a technical file when the MDD 9342EC was not so structured. Despite being named ISO 9000 this template is in fact built with the ISO 9000 family for quality management systems in mind and as such can be used for ISO 9001. GMP Certificate will be issued by a third-party organization after inspection of the facility based on a guideline.
Our core and safety processes as defined in the quality management manual must be reviewed at minimum once per year. ISO 134852016 Section Document Section. He participated in implementation of these standards in more than 100 SMEs through creation of documentation and performing in-house trainings for maintaining management system internal audit and management review. It was just good practice. 424 All 425 All.